Abstract
Introduction:PTPN11 is a proto-oncogene that encodes tyrosine phosphatase with 2 Src-homology 2 domain (SHP-2), a protein tyrosine phosphatase crucial in regulating the RAS/MAPK pathway and hematopoietic cell development. Germline and somatic PTPN11 mutations are associated with Juvenile Myelomonocytic Leukemia (JMML), with inferior outcomes and higher relapse rates than other JMML subtypes. The clinical significance of PTPN11 mutations in AML remains inconclusive, and there is limited information on how PTPN11 mutations impact AML outcomes.
Methods: AML patients (pts) with PTPN11 mutations were identified at New York-Presbyterian/Weill-Cornell Medicine and Vanderbilt University Medical Center. Patients were stratified by age (<60 vs. ≥60 years); log-rank tests were used for survival data and the fisher-exact test was used to compare non-survival data.
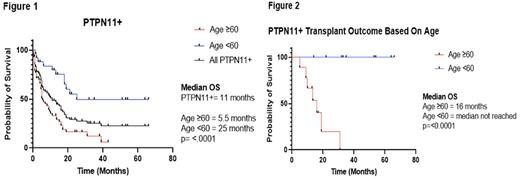
Results: 83 AML pts with PTPN11 with evaluable data were identified. The median age was 63, (32 female, 51 male). Among 83 pts, 74 pts underwent treatment: 33.78% (25/74) pts achieved complete response (CR) at some point during treatment, with induction CR 25.7% (19/74) and re-induction or Salvage CR 10.9% (6/55). In older patients, 30/49 (61.2%) received low-intensity regimens and 18/49 (36.7%) received high-intensity regimens. Venetoclax/hypomethylating agents (HMA) (n=17) were the most commonly used treatment in the low-intensity group. Only 3/17 pts (17.7%) achieved a CR, which is significantly lower than what would be expected based on historical controls. Amongst pts who received high intensity therapy, (all received either 7+3 or CPX-351) 5/18 (27.7%) achieved CR. Out of 25 younger pts, 21 received high-intensity regimens, (4 received low-intensity regimens). CR was achieved in 9/21 pts (42.9%), which is also significantly lower than what would be expected based on historical controls. Median overall survival (OS) of the entire cohort was 11 months (figure 1). Median OS was significantly worse in older pts vs younger pts (5.5mo vs 25.0 mo, p=<0.0001, figure 1). Older pts had significantly decreased total CR rates (24.5% vs 52%, p=0.0221), fewer initial high-intensity regimen (36.7% vs 84%, p=0.0002), more initial low-intensity regimen (51% vs 16%, p=0.002), lower hematopoietic stem cell transplantation (HSCT) rate (15.79% vs 38.46%, p=.046), and lower HSCT survival rate at the time of censor (22.2% vs 100%, p= <0.0007). In older pts there was no significant difference in median OS as a function of higher vs lower intensity induction regimen (5 months vs 9 months, p=.9475). In pts that underwent HSCT, median OS was significantly longer in younger pts than older pts (16 months vs NR, p=<0.0001, figure 2). At time of censor, 2/9 (22%) of older pts who underwent HSCT were alive compared to 10/10 younger pts (100%).
By 2017 ELN risk classification, 16 pts were favorable risk, 20 intermediate, and 47 adverse. Mutational analysis showed co-mutations in DNMT3A 28.9% (24/83), NPM1 27.7% (23/83), RUNX1 22.9% (19/83), ASXL1 15.6% (13/83), NRAS 15.6% (13/83), and TET2 15.6% (13/83). Older pts had significantly increased RUNX1 co-mutations (19 vs 0, p=<0.0001).
Conclusion:PTPN11 mutations were associated with significantly worse OS in older pts. Furthermore, PTPN11 mutations correlated with poor response to high-intensity and low-intensity regimens, including Venetoclax/HMA. In particular, the response to Venetoclax/HMA was worse than what would be expected compared to historical controls. For older pts who achieved a CR and underwent HSCT, survival was uniformly poor. Conversely, the majority of younger AML pts with PTPN11 mutations received high-intensity induction regimens and had significantly better survival outcomes with HSCT than the older age group. These findings suggest that younger pts benefit from high-intensity induction regimens and early consideration of HSCT, while in older pts attempts should be made to focus on clinical trials and novel approaches at targeting the aberrant activated signaling pathways in PTPN11 mutated AML.
Disclosures
Desai:Takeda, Bristol Myers Squibb, Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Research: Research Funding. Roboz:Actinium: Consultancy; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Karyopharm Therapeutics: Research Funding; CTI: Research Funding; Bayer: Consultancy, Other: Travel and accommodation expenses; Agios: Consultancy, Research Funding; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Astellas: Consultancy; Takeda: Consultancy; Array BioPharma: Other: Travel and accommodation expenses; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; Tensha Therapeutics: Research Funding; Bristol Myers Squibb: Consultancy; Roche: Consultancy; Celltrion: Consultancy, Other: Travel and accommodation expenses; Mesoblast: Consultancy; Helsinn Therapeutics: Consultancy; Amgen: Consultancy; Bristol Myers Squibb: Consultancy; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; GlaxoSmithKline: Consultancy; Jasper Therapeutics: Consultancy; Jazz: Consultancy, Other: travel; Clovis Oncology: Other: Travel and accommodation expenses; Mofitt Cancer Center: Research Funding; Amgen: Consultancy, Other: travel; Agios: Other: travel, Research Funding; Onconova Therapeutics: Research Funding; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Sandoz: Consultancy, Other: Travel and accommodation expenses; Daiichi Sankyo: Consultancy; MEI Pharma: Consultancy, Research Funding; Otsuka: Consultancy; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; MedImmune: Consultancy, Research Funding; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses. Ritchie:Celgene: Consultancy; Pfizer: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Takeda: Consultancy. Savona:ALX Oncology: Research Funding; Forma: Consultancy; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; AbbVie: Consultancy, Other: travel expenses; Takeda: Consultancy; Astex Pharmaceuticals: Research Funding; Geron: Consultancy; Novartis: Consultancy; Sierra Oncology: Consultancy, Other: travel expenses; Incyte Corporation: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Taiho Pharmaceutical: Consultancy. Guzman:Samus Therapeutics: Other: Inventor on licensed IP; Seq RX: Current holder of stock options in a privately-held company; BridgeMedicines: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal